Recently, the team of Wang Xiaodong, a researcher at the Research Center of Aerospace Catalysis and New Materials at the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, has made new progress in the pyrolysis of carbon dioxide and water-based solar fuel (syngas or hydrogen) at high temperature. "Energy and Environmental Science" (Energy Environ. Sci.).
The two-step solar high-temperature thermochemical energy storage is a process that uses focused solar energy to thermally crack carbon dioxide and water at high temperatures. This method can convert intermittent, low energy density, unevenly distributed solar energy into stable, high energy density, easy to store and transport solar fuel (syngas or hydrogen), to achieve direct conversion of solar energy to chemical energy; due to its gas-solid The phase operation is simple, and the conversion efficiency of solar energy to chemical energy is high, which has attracted extensive attention from researchers in recent years. Therefore, how to design a catalytic system with excellent performance to achieve efficient activation and conversion of carbon dioxide and water is of great significance and challenging.
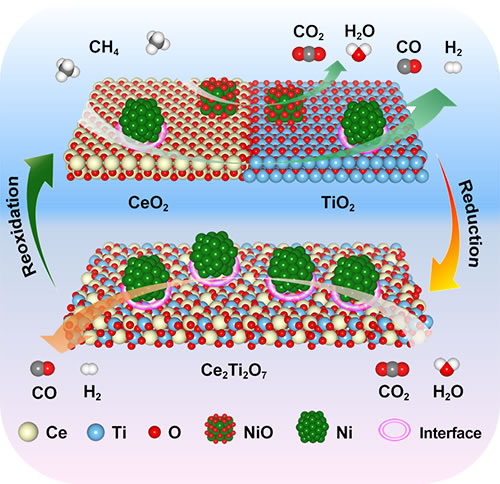
In the preliminary water cracking research work (AIChE J), the team developed a CeO2-SnO2 composite oxide phase change material, which can effectively reduce the thermal reduction temperature of the first step and increase the production of hydrogen. However, the water cracking rate of this method is low, and the hydrogen generation rate and cycle stability need to be further improved. On this basis, the team developed a nickel-based catalyst supported on CeO2-TiO2 composite oxide, and introduced a reducing agent-methane during the first thermal reduction process, which can greatly increase the generation rate and output of solar fuel. The study found that at 900 ° C isothermal conditions, the carbon dioxide and water cracking reaction to generate carbon monoxide and hydrogen production rates were as high as 168.8 and 97.5mLmin-1g-1, which is the highest value reported so far. In addition, the conversion rate of methane partial oxidation is as high as nearly 100%. After 50 redox cycles, the catalyst can still maintain a high carbon dioxide and water cracking rate and methane conversion rate. Detailed characterization and DFT theoretical calculations show that there is a synergistic effect between elemental nickel and CeO2-TiO2 composite oxide: the elemental nickel and nickel / oxide interface are the active centers of the cycle reaction, which can effectively activate the inert gas molecules carbon dioxide, water and methane , To promote the phase change of CeO2-TiO2 composite oxide to Ce2Ti2O7 pyrochlore during the cycle, and the deep reduction of cerium; the intercalation and removal of lattice oxygen of CeO2-TiO2 composite oxide into carbon dioxide and water cracking during the cycle, and Partial oxidation of methane provides a thermodynamic driving force, thereby achieving high activity and high stability of the catalyst. This work provides an important theoretical basis and a new strategy for designing a highly efficient catalytic system for solar thermochemical energy storage technology.
The above research work was supported by the National Natural Science Foundation of China and the Strategic Pilot Technology Special Project of the Chinese Academy of Sciences.
Graphite Sheet,Carbon Graphite Sheet,Thermal Graphite Sheet,Pyrolytic Graphite Sheet
Henan Carbons New Material Technology Co., Ltd. , https://www.hncarbons.com